AREA 4.
Using tripeptides as chelates for radiotherapeutic drug design
Specific Project Aims:
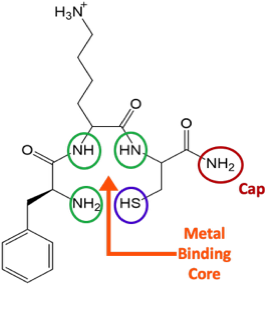
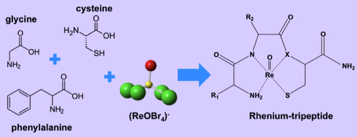
In this project we make use of basic inorganic synthetic techniques coupled with chromatography, spectroscopy and crystallography to develop an understanding of how Re binds to basic tri-peptide sequences. By specifically designing the peptide sequences we can control the NxSy binding motifs and analyze how the changes we make affect the stability and diastereomer distribution of the resulting Re-tripeptide complexes. The knowledge we gain will then be applied to Re-188 tripeptide chemistry for the development of more effective radiotherapeutic drugs
Integrated Materials Development Group
Designing tripeptide chelates with NXSY binding cores to explore the coordination chemistry and stability of the radiometal Re-188
The Probelm.
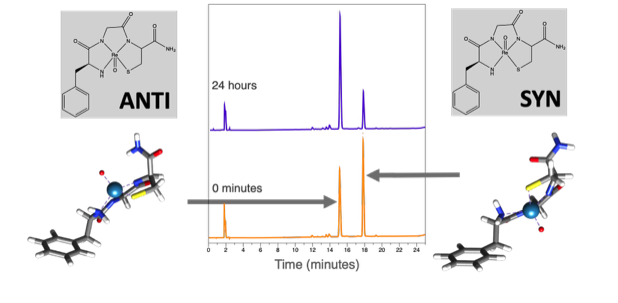
When Re-tripeptides are synthesized as radiotherapeutic drugs, the products exist in 2 diastereomeric forms that can interconvert over time. The biodistribution and efficacy of the radiotherapeutic drugs is often very different for the two diastereomers. While we know that diastereomers can form, there is a limited understanding of how to stabilize one form preferentially over the other and there are many peptide sequences that have yet to be studied.